Arrange the Following in Order of Increasing Rf
Arrange the following in increasing lattice energy. Acetic acid acetaldehyde 2-octanone decane and 1-butanol.
Compounds in the order of increasing polarity.

. Increasing Rf on a TLC plate acetic acid 1-butanol. K_2SO_4 CaCl_2. Explain the reason for the order of difference in rf value.
The more polar the mobile phase the more it interacts with the solute and the more the distance it travels from the baseline. I know the answer. Therefore the order of increasing Rf values for the solvents in the question is.
Thin-Layer Chromatography Post-Lab Exercise 1. If silica is used as adsorbent and hexane is solvent. Do i have to do calculatioNs is there a way to know no matter what.
Hence the order is. Arrange the following in order of increasing R_f on a TLC plate. Decane 2-octanone acetaldehyde acetic acid 1-butanol.
Submitted by Anonymous not verified on Sat 04092011 - 1424. Arrange the following compounds in order of increasing Rf in a TLC analysis. Pressure atm Select the paths that lead to changes in BOTH the average kinetic and potential A.
Benzoic acid nonane 3-heptanone and cyclohexanol. What will be the result of adding too much sample to the. Boiling points increase with increase in size due to higher vander waals forces.
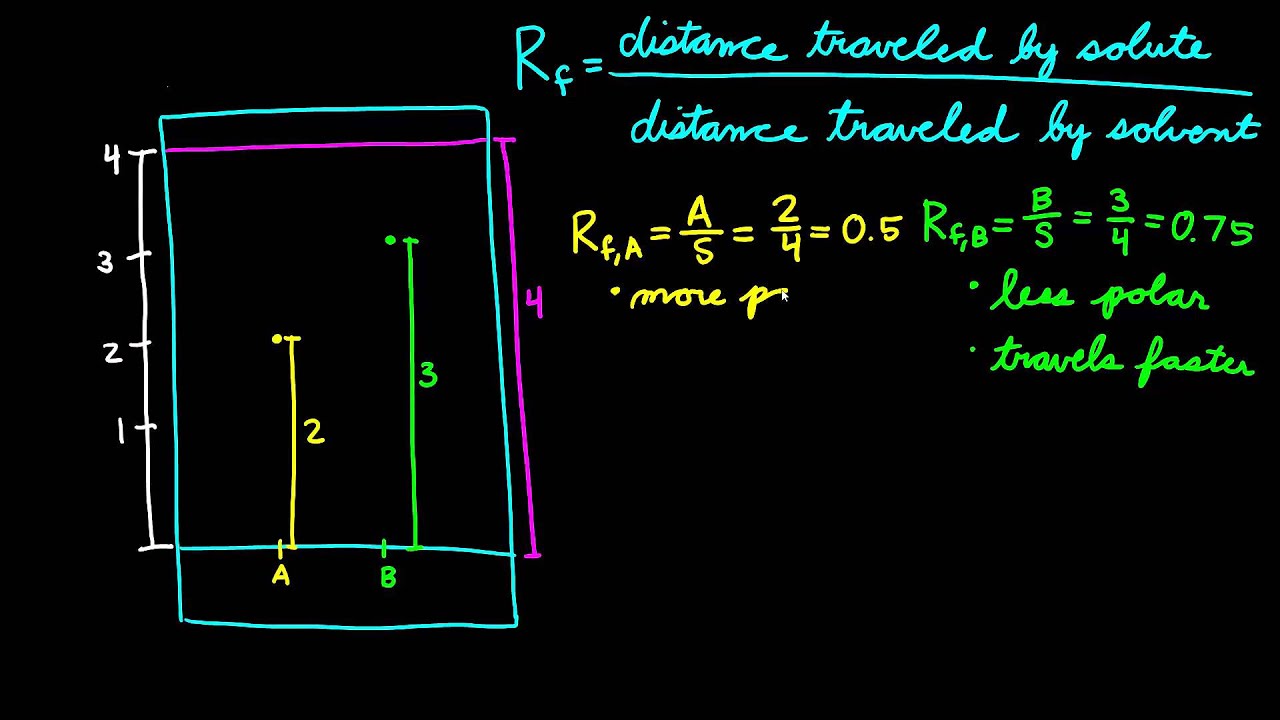
More polar will be smaller Rf because it will stick to plate. C I. 1 point Rf values increase with decreasing polarity of the compound because the more polar the compound the more strongly it stays attached to the stationary phase and travels less distance.
Arrange the following in increasing lattice energy. CH3 CH23COOH CH3 CH26OH CH3 CH210CH3. Decane 2-octanone acetaldehyde 1-butanol acetic acid.
Arrange the following in order of increasing Rf with TLC. Al3 Si Al O2-. Arrange the following compounds in order of increasing Rf in a TLC analysis.
But why is that the answer. Acetic acid acetaldehyde 2-octanone decane and 1butanol. B e C O 3 M g C O 3 C a C O 3 S r C O 3 B a C O 3.
B Cl F Br. Arrange the following in order of increasing Rf on thin layer chromatography. Acetic acid acetaldehyde 2-octanone decane and 1 butanol.
L i 2 C O 3 N a 2 C O 3 K 2 C O 3. Cs The semi-colons indicate a new period row. Why must the spot be applied to the TLC plate above the level of development solvent.
Benzoic acid benzaldehyde 3-heptanone nonane and cyclohexanol. The smallest Rf value would be given by the substance most strongly adsorbed to the silica gel. Arrange the following in order of increasing Rf with TLC.
The atomic radius of these elements and ions will increase in the following order. The elements will have the atomic radius according to their atomic number and their respective placement in the periodic table. Rf value of any compound on TLC or paper chromatography or any other form of chromatography depends on Stationary phase Mobile phase polarity of compound temperature of TLC system Saturation of solvent in close environment and.
Arrange the following compounds in order of increasing rF in a TLC analysis. Arrange the following compounds in order of increasing Rf value on silica gel. RI RBr RCl RF.
The path that leads to change in both average kinetic and potential energy of the system is iii fa. See below for O-2. Arrange the following compounds by increasing RF value and determine polarity.
Hence the compounds in the order o View the full answer. Arrange the following electrolytes in the increasing order of coagulation power for the coagulation of As_2S_3 sol. A P N O.
This problem has been solved. Rf distance traveled by the compound distance of the solv View the full answer Transcribed image text. Arrange the following in order of increasing Rf on thin-layer chromatography.
Rf value decreases with the increase in the polarity of the compounds. See the answer See the answer done loading. Arrange the following compounds in order of increasing Rf in a TLC analysis.
Benzoic acid benzaldehyde 3-heptanone nonane and cyclohexanol. Atomic size decreases left to right on the periodic table because the attractive force of the protons on the same number of rings of electrons increases for each element as we move left. View postlab23pdf from PHYS 1111K-1112 at Georgia State University.
Benzoic acid benzaldehyde 3-heptanone nonane and cyclohexanol. Increasing order of the elements with respect to the radii is. Octanoic acid butyraldehyde 2-octanone decane and 1-pentanol.
Solution for Sort the following molecules in order of increasing Rf value assuming a normal-phase silica TLC plate was used with a 101 hexanes. If the solvent does not evaporate from the top of the plate the Rf values should not be affected. Acetic acid acetaldehyde 2-octanone decane and 1-butanol.
Arrange the following in order of increasing Rf on thin-layer. The order of increasing RF values in TLC is Benzil methanol anthracene and tryphenyl. As given in the option A the Phosphorous is the element present right.
The radii of the elements increase from left to right in the table. Thus high solvent polarity leads to higher Rf value.

Instahibit Retractable Electric Projection Screen 92 In 2022 Projection Screen Installation Design Ceiling Installation

Periodic Trends Chemwiki Electron Affinity Ionization Energy Chemistry Textbook

Pin By Rajesh Nambiar On Smac Visualizations Big Data Marketing Infographic Marketing Data Driven Marketing
No comments for "Arrange the Following in Order of Increasing Rf"
Post a Comment